13 Dec 2024
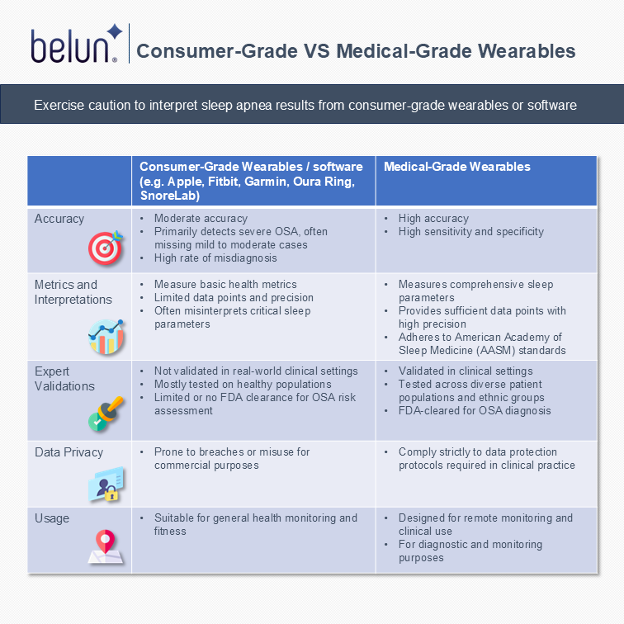
Apple recently introduced a feature for the Apple Watch that alerts users to potential obstructive sleep apnea (OSA), helping to raise awareness of sleep health, particularly among individuals without noticeable symptoms. However, there are important considerations for consumers using consumer-grade wearables or software as medical devices (SaMD) to detect sleep apnea:
1) Limited Accuracy for clinical diagnosis
Consumer-grade wearables, including the Apple Watch, often exhibit moderate accuracy suited for general health monitoring rather than clinical diagnosis. For example, the Apple Watch can only detect severe OSA, potentially missing individuals with mild or moderate OSA and providing false reassurance. Even detected cases of severe OSA may not always be accurate, leading to potential misdiagnosis.
2) Restricted Metrics and Misinterpretations
These devices typically measure basic health metrics with limited data points and precision. They often inaccurately derive critical sleep parameters based on standards from the American Academy of Sleep Medicine (AASM). For smartphone acoustic apps, users should note that sleep measurements can be compromised by the presence of bed partners or pets sharing the same room. This can result in misinterpretations and confusion when physicians attempt to explain inconsistencies in wearables or software-derived reports to patients.
Many wearable devices and sleep apps track basic health data but may not be very accurate. They often estimate important sleep details using methods that don’t always follow trusted medical standards, which can lead to errors.
For smartphone apps that use sound (acoustics) to measure sleep, keep in mind that the presence of a bed partner, pets, or even background noises in the room can interfere with the results. This can lead to confusion, especially when doctors try to explain why the app’s reports don’t match your actual sleep patterns.
3) Limited Validations and Limitations
Caution is necessary when relying on consumer-grade wearables or even FDA-cleared SaMD for sleep assessment. Most are validated only in healthy populations, not in real-world clinical settings. While some devices or apps might be FDA-cleared, they are typically limited to assessing the risk assessment of OSA, not providing a definitive diagnosis.
4) Data Privacy Concerns
Health data collected by wearables may be vulnerable to breaches or misuse due to commercial considerations. Many consumer-grade devices and apps do not adhere to strict data protection protocols required in clinical practice, raising concerns about privacy.
5) Restricted to General Health and Fitness Usage
Consumer-grade wearables or SaMD should be regarded primarily as tools for general health monitoring and fitness tracking. Individuals experiencing sleep issues are strongly advised to undergo a proper sleep test for an accurate medical diagnosis.
Ultimately, while consumer wearables can increase awareness of sleep health, they are not a substitute for professional medical diagnosis and care.
What Belun is capable of ?

The Belun® Ring stands as the only FDA-cleared wearable for sleep stage classification within OSA diagnosis, achieving a high accuracy rate of 89% for moderate OSA and above. The Belun® Ring demonstrates impressive performance in sleep stage classification, achieving accuracy rates of 88% for Wake, 82% for REM sleep, and 90% for NREM sleep.(1) The device shows excellent sensitivity (91%) and specificity (88%) in detecting moderate to severe OSA.(1)
Extensively validated across diverse patient populations(1–4), the Belun® Ring has proven reliable for clinical and research applications in sleep medicine and is used by over a hundred organizations locally in Hong Kong and globally. This includes universities, academic research units, hospital authorities, clinics, dental offices, and elderly centers. Internationally, the Belun Sleep System has expanded to Asia (Singapore, Thailand, Malaysia, Taiwan, Indonesia, Australia), the U.S., and Latin America (Mexico) for both clinical and research purposes.

Partnering with Belun :
Up to now, over a hundred organizations, including HK hospital authority hospitals, medical groups, clinic groups, dentists, and elderly centers, have selected to use the Belun Sleep System, Belun Ring, and Belun® remoVital monitoring system. Many doctors read our medical journal papers, including:
1) Strumpf Z, Gu W, Tsai CW, Chen PL, Yeh E, Leung L, et al. Belun Ring (Belun Sleep System BLS-100): Deep Learning-Facilitated Wearable Enables OSA Detection, Apnea Severity Categorization, and Sleep Stage Classification in Patients Suspected of OSA. Sleep Health. 2023 Aug;9(4):430–40. (https://journals.lww.com/jhypertension/Abstract/2023/06000/The_Belun_sleep_platform_to_diagnose_obstructive.16.aspx),
2) Gu W, Leung L, Kwok KC, Wu IC, Folz RJ, Chiang AA. Belun Ring Platform: a novel home sleep apnea testing system for assessment of obstructive sleep apnea. J Clin Sleep Med. 2020 Sep 15;16(9):1611–7.(https://jcsm.aasm.org/doi/10.5664/jcsm.8592),
3) Ou YH, Ong J, Thant AT, Koo CY, Leung L, Sia CH, et al. : The Belun Sleep platform to diagnose obstructive sleep apnea in patients with hypertension and high cardiovascular risk. J Hypertens. 2023 Jun;41(6):1011–7. (https://pubmed.ncbi.nlm.nih.gov/37071415/)
4) Yeh E, Wong E, Tsai CW, Gu W, Chen PL, Leung L, et al. Detection of obstructive sleep apnea using Belun® Sleep Platform wearable with neural network based algorithm and its combined use with STOP-Bang questionnaire. PLoS ONE. 2021;16(10):e0258040. (https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0258040)
If you would like to know more about how to adopt Belun’s solution in your organization, please feel free to contact us to schedule a meeting by filling out the form below: